
Press Release - Mobile MIM
Mobile MIM, First FDA-Cleared Diagnostic Medical Imaging App, Now Available on the U.S. App Store
Cleveland, Ohio – February 15, 2011 – MIM Software Inc., a leading global provider of medical imaging software, announced today that Mobile MIM is available on the U.S. App Store. This news follows the FDA’s announcement on February 4 that the app had received 510(k) clearance for remote diagnostic viewing of CT, PET, MRI, and SPECT images on the iPhone®, iPod touch®, and iPad®.
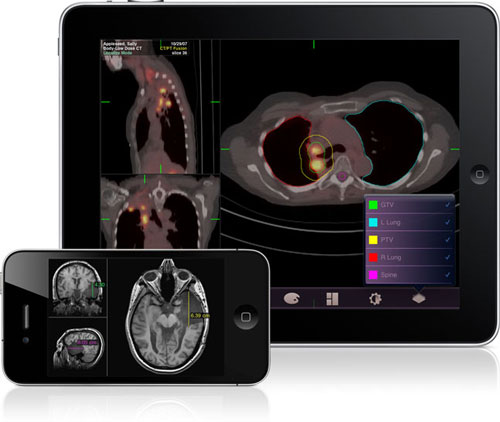
The Mobile MIM App is free to download and includes sample images to demonstrate its functionality. Physicians and other medical professionals can download images to the device using MIMcloud.com, an Internet-based service which allows secure upload and download of encrypted medical data. Alternatively, a MIM workstation can be used at a facility to transmit the images to Mobile MIM. In either case, Mobile MIM is HIPAA compliant and indicated for use only when the physician does not have access to a workstation.
"The FDA's decision to take an active interest in medical imaging apps being marketed to physicians is an important step for the future of medicine," says Mark Cain, CTO of MIM Software. "The app gives physicians and doctors anytime, anywhere access to important scans, raising the bar for efficiency, convenience, and patient care."
The Mobile MIM App is designed as a thick client, which means the data is downloaded to the device for viewing instead of being streamed to the device from a server. To secure the protected health information, Mobile MIM provides “at rest” encryption through the use of a passcode and 128-bit AES encryption from within the app. All transfers use SSL encryption.
Mobile MIM features data set interaction using standard tools, such as zoom, pan, window and level, and it displays volumetric data with multi-planar reconstruction. Mobile MIM allows the physician to measure distance and intensity values, annotate, and display regions of interest.
“We strive to create products that enhance patient care,” says Cain. “We understand that some may see this as a way for doctors to cut corners in favor of convenience. However, physicians have to answer for the decisions they make, and their choices are carefully considered. Before today, doctors who needed to see images but could not get to a workstation had to opt for a less preferable course of action or no action at all. When my health is in question, I don’t want my care compromised because of the doctor’s location.”
Regarding the availability of Mobile MIM only on Apple devices, Cain said, “The iOS platform has proven itself with its combination of massive market penetration and a lineup of devices that are both tightly controlled in their manufacture and consistent in their characteristics. Without the steady consistency and quality of Apple products, we could not have sustained the scrutiny of the prolonged regulatory process.”
Mobile MIM is intended for use by medical professionals, not patients. The company plans to release a second app intended for patients, VueMe. On July 5, 2010, MIM Software submitted a 513(g) request to the FDA to inquire about the classification of a patient image viewing iPhone app, and on September 3, 2010, the FDA determined that it would be a medical image storage device, a FDA class I medical device.
The Mobile MIM App is available for free from the U.S. App Store on the iPhone, iPod touch, and iPad,or at iTunes.
About MIM Software Inc.
MIM Software Inc. provides practical imaging solutions in the fields of radiation oncology, radiology, nuclear medicine, neuroimaging, and cardiac imaging. MIM offers solutions for PC and Mac workstations, as well as mobile iOS and cloud-based platforms. MIM is a privately held company that sells its products globally to imaging centers, hospitals, specialty clinics, research organizations, and pharmaceutical companies.
For more information, email info@mimsoftware.com.





 京公网安备11010802039423号
京公网安备11010802039423号